Advantages of Softened Water
Softened water leaves the water feeling silky and luxurious and could leave laundry brighter and softer. It can also help to reduce the amount of detergent needed for a wash.
Other advantages of water softening include cleaner glasses and dishes, softer hair after washing, a reduction in scale in bathrooms as well as saving money by reducing the cost of repairs, maintenance in household fixtures such as heaters, showers and boilers.
What is ‘Hard Water’?
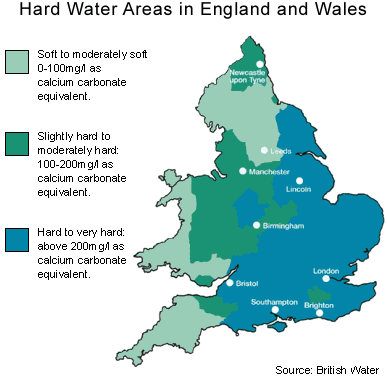
Hard water is formed when rainwater passes through chalk and limestone rocks, absorbing calcium and magnesium minerals.
In an estimated 60% of UK homes, the mains water supply is ‘hard’, with Eastern, Central and Southern UK water defined as ‘hard to very hard’, due to the geology of these regions.
The minerals contained in hard water settle out as a deposit of hard scale, wherever the water is heated or when cold standing water evaporates. Examples of this include; white marks, stains and scale on sinks, baths, toilet bowls and around the base of taps, blocked showerheads, scale deposits on kettles and water heating elements and clogging of pipe work.
Types of Softener
Ion-exchange water softening technology is well advanced with a variety of softeners offering various features and benefits.
Your local supplier will provide guidance on the most suitable ion-exchange unit for your needs. You may be offered cheaper ‘physical’ devices which claim to work using magnetic, electrolytic or electronic means. Whilst in some circumstances they may reduce the formation of scale in heating systems, they do not soften the water.
When the British Water, Quality Water Group studied Physical Conditioners, it concluded:
- Magnetic, electrolytic or electronic devices … do not change the chemical composition of the water
- There is no widespread agreement, even among those manufacturing the devices, on how these work
- Even when a scale reducer works very well in one home, it may fail to work in other homes, even though the conditions appear to be similar
- Companies selling scale inhibitors should provide an extended-time, money-back guarantee to protect the customer should the device fail to work in their home
- Scale inhibitors only reduce scale formation and, as it has no effect on scum formation, it will not reduce the amount of soaps, shampoos and household cleaning products used.
For this reason, we recommend that you choose an ion-exchange water softener – one that uses salt to remove the hardness. Only an ion-exchange unit can ensure that the hardness is removed.
How Do Water Softeners Work?
Ion-exchange water softeners work by taking the minerals that cause hardness out of the water. The softener contains millions of tiny resin beads which remove calcium and magnesium as the water passes through. The resin attracts and traps these minerals so that only softened water enters your plumbing system. Periodically, a timer automatically flushes the resin clean with brine to regenerate it.
A domestic ion-exchange water softener is the only process specifically designed to completely remove all hardness from the mains water supply. Water softened in this way will also gradually remove existing scale from pipe work, bathroom fittings and heating elements.
Other electronic and magnetic devices do not remove the hardness from the water. Some may have some effect in reducing or inhibiting hardness scale, but they will not soften the water.
Only with an ion-exchange softener will you enjoy all the benefits of softened water in your home.
Why Salt?
Salt is used to make brine to flush clean, or ‘regenerate’, the resin beads.
Always use a high purity salt product specifically designed for use in water softeners. Fine cooking or table salt could interfere with the performance of the softener, whilst coarse sea or rock salt may contain impurities.
It is particularly important to use an ‘ion-exchange’ water softener – that is one that uses salt to cleanse the resin which captures the hardness minerals. Devices claiming to use magnetic or electrical forces may be cheaper, but will not remove the hardness.
Water Softening & the Environment
About 60% of the UK has a water supply that is ‘hard’. This means it contains relatively high levels of calcium and magnesium in the form of carbonates, resulting from rainwater leaching through limestone rock.
The most effective way to overcome the effects of hard water is to soften using an ion exchange water softener. Whilst the use of ion exchange water softening systems does have an environmental impact, this is more than outweighed by the benefits in energy savings and reduced consumption of detergents and other chemicals.

Environmental Benefits
Whilst not a comprehensive list, some of the substantiated benefits of using softened water are listed below:
Energy Consumption
Work published in France shows that a 1mm increase in scale thickness can reduce heating efficiency by an average of 6%, with the first 0.4mm showing a rate as high as 10%.
Another study by New Mexico State University, showed that over 29% more energy was used by gas heaters run on hard water, and 21% more with electric heaters.
Life Cycle Analysis (LCA)
As yet, analyses of the full life cycle of water-based processes are very limited. However, a Swiss study on domestic clothes washing showed that ion exchange softening was environmentally better than either the use of unsoftened water, or captured rainwater.
Detergent & Cleaning Agent Consumption
The Association Français pour l’Etude des Eaux found soap and detergent use more than doubled in hard water. Since then detergent formulations have been adapted to take into account hard water, by the use of additives such as phosphates, yet many brands still indicate considerably different usage rates for hard and soft water.
Both the additional detergent and the ‘softening’ additives increase the environmental burden of laundry and bathing activities.
Because of its scaling effects on sanitary ware, anti-scalants are often necessary in hard water areas. These tend to be acid based, so are both costly and have an environmental impact.
Fabric life
A study carried out in a Chicago YMCA laundry, found that softening the water supply increased the life of frequently washed household items such as sheets, pillow slips and bath towels by 20-40%.
Environmental Impact of Softening
Raw material consumption
Softening equipment is manufactured from various materials, including common metals and plastics, which are recyclable.
It should also be noted that the equipment has a long life, typically over 15 years, and requires minimal maintenance.
Salt (sodium chloride) is the only consumable product used in the process. Salt is abundant in nature, in both geological deposits and the sea. It is relatively easy to extract and process, with little chemical use or impact on the landscape.
The principal environmental impact is the energy used in evaporation, which can be minimised by the use of efficient evaporator systems.
Salt Discharge
The regeneration cycle of the softening process discharges a quantity of sodium chloride into the drainage system, and sodium ions are added to the softened water.
Studies on septic tanks and sewage systems have repeatedly shown that the sodium/salt burden has no harmful effect. Sodium does not bio-accumulate which ensures that there are no long term environmental effects.
Modern equipment is designed to have a high salt efficiency, so effects on the overall chloride and sodium environmental burden is minimised.
Water consumption
The regeneration and backwash cycles of the softener do use more water than standard consumption. However, modern demand-driven equipment keeps wastage to a minimum.

Soft Water & Metal Corrosion
Because naturally soft water has been associated with increased corrosion in water supply systems, there has been concern that softened water might have a negative impact on metal.
However, tests carried out by the US Environmental Protection Agency have clearly demonstrated that there is no significant difference in corrosion performance between softened water and its source water. Indeed there were indications that in some cases softened water may be less corrosive.
References:
Le Grand Livre de l’Eau
M A Bernardis, La Manufacture 1990
Softened Water Energy Savings Study – Controlled Experimental Testing Program on Household Water Heaters
Engineering Experiment Station, New Mexico State University, Las Cruces, New Mexico, 1979
La Dureté de l’Eau Intérêt Économique de l‘Adoucissement Collectif Pour l‘Usager.
Etude de synthèse de l’agence de l’eau Artois-Picardie
Association français pour l’étude des eaux 1981
Benefits of Using Soft vs Hard Water in Laundering Operations
R W Peters, C M Ladisch, Research report sponsored by Water Quality Association (USA) October 1990
The Effect of sodium discharge from water softeners into the septic fields of New Jersey
JCF Tedrow, Rutgers University 1997
Life Cycle Assessment of Rainwater Use for Domestic Needs
V Bronchi, O Jolliet & P Crettaz
Ecole Polytechnique Federale de Lausanne, Institute of soil and water management. Paper given at 2nd Inter-Regional Conference on Environment-Water 1999
Ion Exchange Softening: Effects on Metal Concentrations.
TJ Sorg, MR Schock, and DA Lytle. Water Supply and Water Resources Division, National Risk Management Research Laboratory, Office of Research and Development, USEPA, Cincinnati, Ohio.
Journal AWWA, 91(8):85-97 1999
Potential Effects of Water Softener Use on Septic Tanks Soil Absorption On-site Waste Water Systems
Corey, Tyler and Holotoo University of Wisconsin- Madison
