Chlorine & Nature
It is fundamental to the life of plants and animals. In our bodies, chlorine, as hydrochloric acid, helps break down food for digestion. It is also part of the immune system that protects us from infection.
Nature and chlorine can do some remarkable things like making a pain killer 200 times more powerful than morphine but with no side-effects – all part of the natural defences of an Ecuadorian tree frog!

A Power of Good
Perhaps it is not surprising that so many man-made chlorine products come from naturally good ideas. The very first were disinfectants to combat the spread of disease.
Chlorine was first introduced into drinking water late in the 19th century to control the spread of waterborne diseases like cholera and typhoid – disease that have killed more people than all the wars in human history.
Continuing to fight diseases is just as important today. Up to fifteen million people still die every year from the effects of drinking untreated water.
Chlorine is used in many other ways to make a huge range of products. The secret is reactivity – chlorine’s ability to bond easily with other chemical elements. This makes chlorine one of the most successful chemical building blocks in industry and in nature. Over 2000 natural organic chlorine compounds are at work in the environment.
Keeping Pace… Keeping in Touch
Far from being the thing of the past, many industries have turned to chlorine chemistry to find new ways of doing things.
For example, high performance plastics and polymers made with chlorine have replaced scarce natural materials like hardwoods in the modern school, office and home. It is used to make computer equipment (including, most likely, the device you’re using to read this page), televisions, CDs, tennis racquets, shoes, skis, car parts, telephones, satellites… the list is almost endless.
Chlorine is an asset in the modern world of fashion, used in the production of modern fabrics as well as in the dry-cleaning process.
Essentials for Life
In the modern world, as in nature, chlorine helps to sustain life and protect our health.
Chlorine based water treatments are among the most effective available and the only ones that keep working right up to the tap. The same powerful disinfecting properties are found in hygiene products used in hospitals and homes everywhere. Preventing the spread of infection is particularly important in hospitals and chlorine is used to produce sterile packaging, disposable equipment and even saline drips and blood bags.
Modern medicine relies on chlorine in other ways too. 85% of pharmaceuticals contain chlorine or are made with it including treatments for heart disease, leukaemia, arthritis and allergies.
Chlorine is also at work in agriculture – but without it, people still live in near famine in many parts of the world. 96% of the products used to control pests, crop diseases and weeds are based on chlorine. The result is high quality, high yield crops and lower food prices.
Why is Electrolysis Useful?
.. and some common uses
All three products, products: chlorine (Cl2), sodium hydroxide (NaOH), and hydrogen (H2) are useful individually but can also be combined to make new important compounds.
Sodium hydroxide and chlorine can be made into sodium chlorate (l). This is a strong oxidising agent and is excellent at killing bacteria – but is can be explosive when mixed with the wrong chemicals. Sodium chlorate is used as the active ingredient in a wide variety of commercial herbicides. The main commercial use for sodium chlorate is for making chlorine dioxide (ClO2) used for the bleaching of pulp.
Hydrogen and chlorine react together to form hydrogen chloride. This is made into hydrochloric acid by dissolving it in water. Hydrochloric acid made in this way is very pure and can be used safely in the food and pharmaceutical industries.
An important downstream product which helps protect us in our daily lives is sodium hypochlorite (NaClO) which is commonly used as household bleach, used as a disinfectant and in water chlorination. Today the preferred method for the large scale production of sodium hypochlorite is known as the Hooker process (named after Hooker Chemicals, now Occidental Petroleum). In the process, sodium hypochlorite (NaClO) and sodium chloride (NaCl) are formed when chlorine is passed into cold and dilute sodium hydroxide solution. It is prepared industrially by electrolysis with minimal separation between the anode and the cathode. The solution must be kept below 40 °C (by cooling coils) to prevent the undesired formation of sodium chlorate.
Cl2 + 2NaOH →NaCl + NaClO + H2O
Hence, chlorine is simultaneously reduced and oxidized; this process is known as disproportionation.
Hydrolysis
Hydrolysis is a reaction involving the breaking of a bond in a molecule using water. The reaction mainly occurs between an ion and water molecules and often changes the pH of a solution. In chemistry, there are three main types of hydrolysis: salt hydrolysis, acid hydrolysis, and base hydrolysis. http://en.wikipedia.org/wiki/Hydrolysis
Copyright © 2014 CIEC at the University of York, York, UK
The Chemistry of Chlorine
Chlorine, along with its important by-product, sodium hydroxide, is produced from the readily available starting material, rock salt (sodium chloride). It is well known for its use in sterilizing drinking water and in particular swimming pool water.
However, most chlorine is used in the chemical industry in the manufacture of other products. Sometimes chlorine is in the product molecule but on other occasions it is used to produce intermediates in the manufacture of products that do not contain chlorine and the element is recycled.
Uses of Chlorine
The largest use is in the manufacture of poly(chloroethene), PVC. Other major polymers produced using chlorine include the polyurethanes . Although chlorine does not appear in the polyurethane molecule, chlorine is used to make the intermediates, the isocyanates.
The oxygenates (Figure 1) are principally epoxypropane and propane-1,3-diol, which are used to make polyols. These, like the isocyanates, are used in turn to make polyurethanes.
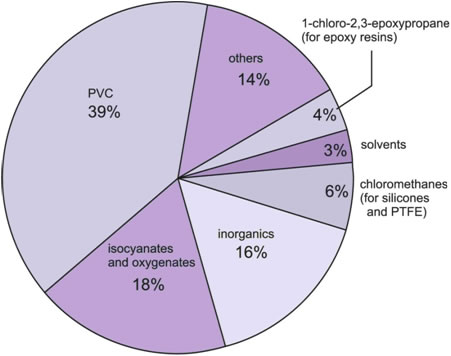 Figure 1 The uses of chlorine.
Figure 1 The uses of chlorine.
1-Chloro-2,3-epoxypropane has many industrial uses, the most important being in the manufacture of the epoxy resins. Among the uses of the chloromethanes are the manufacture of silicones and poly(tetrafluoroethene), PTFE.
The solvents (including trichloroethene) are used in dry cleaning.
Chlorine is also used in the manufacture of many inorganic compounds, notably titanium dioxide and hydrogen chloride.
Most chlorine is produced on the site on which it is going to be used, for example, to make hydrochloric acid and the other compounds described above.
 Figure 2. Although much rock salt is pumped to the surface as brine,
Figure 2. Although much rock salt is pumped to the surface as brine,
some is mined, as is being done in this large underground deposit in Cheshire.
However, some chlorine needs to be transported for example, when it is to be used to purify water. For this, the chlorine is dried by passing it through concentrated sulfuric acid and then compressed and liquefied into cylinders, ready for transportation.
Annual Production of Chlorine
| World | 56 million tonnes |
| Europe | 16 million tonnes |
| North America | 11 million tonnes |
Manufacture of chlorine
Most chlorine is manufactured by the electrolysis of sodium chloride solutions. The other main commercial product is sodium hydroxide. The primary raw material for this process is rock salt (sodium chloride), available worldwide usually in the form of underground deposits of high purity. It is pumped to the surface with high pressure water as a concentrated solution. This solution is often called brine.
A solution of sodium chloride contains Na+(aq) and Cl-(aq) ions and, from the dissociation of water, very low concentrations of H+(aq) and OH-(aq) ions. During the electrolysis of the solution, chlorine and hydrogen gases are produced

As the hydrogen ions are discharged, more water dissociates forming more hydrogen and hydroxide ions. This results in a gradual build-up of the concentrations of hydroxide ions around the cathode, thus producing a solution of sodium hydroxide.
The essential requirement is to maintain an effective and economic means of separating the anode and cathode reactions so that the products, chlorine and caustic soda, will not react to form sodium hypochlorite. This separation has been achieved historically by the mercury amalgam and diaphragm processes.
However, these are being phased out and most new plants use ion exchange membranes, which are the most environmentally and economically sound means of chlorine production.
| (a) Cation exchange membrane cell |
| The cation exchange membrane does not allow any gas or negative ions to flow through it but it allows Na+ ions to move between the brine and caustic compartments. |
| (b) Mercury amalgam cell |
| In the flowing mercury cathode process sodium ions are discharged in the form of a mercury sodium amalgam and chloride ions are converted to chlorine. The amalgam flows to a totally separate compartment, the decomposer (denuder) in which it reacts with water to yield sodium hydroxide solution and hydrogen gas. |
| (c) Percolating diaphragm cell |
| A percolating diaphragm, usually of asbestos, allows a through flow of brine from anode to cathode. It separates the chlorine and hydrogen gas spaces. The migration of OH- ions from the cathode to the anode is prevented by the velocity of liquid flow against them. |
Table 1 The key features of the three electrolytic processes.
Scroll down for more details on these 3 elements.
(a) Cation exchange membrane cell
The anodes are made of titanium coated with ruthenium dioxide. The cathodes are nickel, often with a coating to reduce energy consumption. The anode and cathode compartments are completely separated by an ion-permeable membrane (Figure 3). The membrane is permeable to cations, but not anions; it allows the passage of sodium ions but not chloride or hydroxide ions.
Sodium ions pass through in hydrated form (Na.xH2O)+ so some water is transferred, but the membrane is impermeable to free water molecules.
The sodium hydroxide solution leaving the cell is at ca 30% (w/w) concentration. It is concentrated by evaporation using steam, under pressure, until the solution is ca 50% (w/w), the usual concentration needed for ease of transportation and storage.

Figure 3 The membrane cell.
The membrane (0.15-0.3 mm thick) is a co-polymer of tetrafluoroethene ((Unit 66) and a similar fluorinated monomer with anionic (carboxylate and sulfonate) groups.
(c) The percolating diaphragm cell
In the diaphragm cell (Figure 6), the anodes are titanium coated with a precious metal oxide and the cathodes are steel. There is a porous asbestos diaphragm to separate chlorine and hydrogen that are liberated during electrolysis.
The hydroxide ions formed in the cathode compartment, together with the sodium ions, produce a solution of sodium hydroxide.
The electrolyte level is maintained higher in the anode compartment so that the brine percolates through the diaphragm into the cathode section from where it flows out of the cell with the sodium hydroxide solution.

Figure 6 The diaphragm cell.
The chlorine formed on the anodes rises and is led away.
The cathode solution contains about 10-12% (w/w) sodium hydroxide and 15% (w/w) sodium chloride. This is evaporated to one-fifth of its original volume when the much less soluble sodium chloride crystallizes to leave a solution containing 50% (w/w) sodium hydroxide and less than 1% (w/w) sodium chloride.
(b) The mercury cell
Typical modern gas-tight, rubber-lined or PVC-lined steel cells (Figure 4) are used, which measure about 2 m x 15 m. They have a slightly sloping base over which flows a thin layer of mercury, acting as a cathode. The anodes are a series of titanium plates coated with a precious metal oxide layer, and positioned about 2 mm from the cathode. The cells typically operate in series of approximately 100.
Purified, saturated brine (25% (w/w) sodium chloride solution) at typically 333 K flows through the cell in the same direction as the mercury. This high salt concentration and the anode coating ensures the oxidation of chloride ions rather than that of water which would yield oxygen at the titanium anodes.

Figure 4 The mercury cell and decomposer.
The chlorine is led off as shown in Figure 4.
At the mercury cathode, sodium ions are discharged in preference to hydrogen ions due to the high overvoltage of hydrogen. The sodium forms an amalgam with the mercury. The amalgam contains approximately 0.3% (w/w) sodium.
It moves on to a decomposer cell situated alongside the mercury cell.
The exit brine, containing typically 15-20% (w/w) sodium chloride, is freed of chlorine by blowing air through it, or subjecting the solution to a vacuum. The solution is resaturated with sodium chloride and returned to the cell.
The decomposer cell (Figure 4) is made of steel and contains graphite blocks fixed in the flow of amalgam. Alternatively, the decomposer is a tower packed with graphite spheres. The decomposer acts as a short circuited cell. At the anode sites, sodium is oxidized and the ions pass into solution. At the cathode sites, hydrogen is discharged.
The mercury is returned to the electrolysis cell and the hydrogen passes out of the decomposer. A 50% (w/w) solution of sodium hydroxide is produced in the decomposer and most of it is sold in this form. Some is concentrated by evaporation to 75% (w/w) and then heat ed to 750-850 K to obtain solid sodium hydroxide.
 Figure 5 Chlorine being manufactured using mercury cells. Usually, about 100 cells operate in series. Great care is taken to prevent loss of mercury.
Figure 5 Chlorine being manufactured using mercury cells. Usually, about 100 cells operate in series. Great care is taken to prevent loss of mercury.
By kind permission of Arkema.
